The Early Development of Flibanserin
My journey into the history of Flibanserin starts in the late 1990s. This period marked the birth of Flibanserin, initially developed by the German pharmaceutical company, Boehringer Ingelheim. The drug was initially intended to be an antidepressant, focusing on the treatment of depressive disorders. However, during the clinical trials, it was found that while Flibanserin had minimal impact on depression, it had a significant effect on premenopausal women struggling with Hypoactive Sexual Desire Disorder (HSDD).
HSDD, for those who might not know, is a condition characterized by a persistent or recurrent deficiency or absence of sexual fantasies and desire for sexual activity. While Flibanserin did not cure depression, its potential to tackle HSDD was a significant breakthrough. This unexpected finding set the stage for the drug's future development.
The Shift in Flibanserin's Direction
Once the potential of Flibanserin as a treatment for HSDD was discovered, Boehringer Ingelheim shifted its focus. Instead of treating depression, the company decided to explore Flibanserin’s potential to tackle HSDD. It was a daring move, considering the drug's initial purpose. Yet, the company was hopeful of the drug's potential to improve the quality of life for many women.
Several clinical trials were carried out to test the drug’s efficacy in treating HSDD. Results from these trials were promising, showing notable improvement in sexual desire and decrease in distress among women who used the drug. This served as a green light for the company to proceed with its new direction.
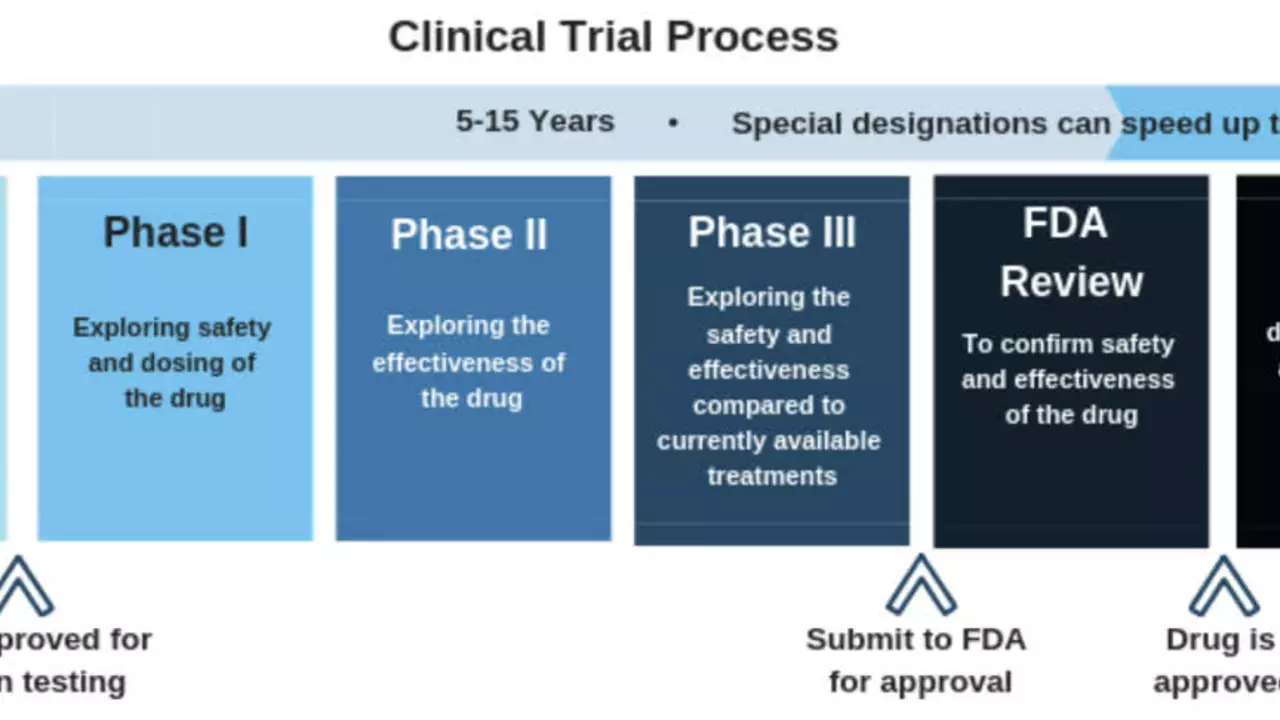
The Rocky Road to FDA Approval
The journey to FDA approval was anything but smooth. Flibanserin first sought approval from the FDA in 2010. However, the FDA refused to approve the drug, citing concerns about its side effects which included nausea, dizziness, and sleepiness. The agency also questioned the drug’s effectiveness and required more robust evidence to prove its benefits outweighed its risks.
Despite the setback, Boehringer Ingelheim did not give up. The company sold the drug to Sprout Pharmaceuticals, a company that took up the challenge to further refine the drug and seek FDA approval.
Sprout Pharmaceutical's Role in Flibanserin's Approval
Sprout Pharmaceuticals began the task of refining Flibanserin and conducting further trials. The company worked tirelessly to address the FDA's concerns, particularly regarding side effects. In 2013, the company resubmitted its application to the FDA, hopeful that the agency would reconsider its initial decision.
Two years later, in 2015, the FDA finally granted approval for Flibanserin, marking it as the first drug approved to treat HSDD in premenopausal women. The approval was a significant milestone, not just for Sprout Pharmaceuticals and Boehringer Ingelheim, but for the millions of women who could potentially benefit from the drug.
The Impact of Flibanserin on Women's Health
Since its approval, Flibanserin has been a game-changer for women's health, particularly in the area of sexual health. The drug, sold under the brand name Addyi, has helped numerous women manage HSDD, improving their sexual desire and reducing distress. It has given hope to women who previously had no pharmacological treatment options available.
While Flibanserin may not have started as a drug intended to treat HSDD, its journey and eventual FDA approval have had a significant impact on women's health. The story of Flibanserin serves as a reminder that sometimes, unexpected breakthroughs can lead to major advancements in medicine and health.

9 Comments
Rama Hoetzlein
July 1, 2023 AT 20:28Flibanserin’s saga is just a textbook case of pharma hubris masquerading as progress 🤬. They chased a “miracle” for women while ignoring the ugly side‑effects, proving that big‑biz ethics are as thin as the drug's efficacy.
Lorena Garcia
July 1, 2023 AT 21:33While the criticism isn’t unfounded, the drug did give a voice-and a treatment option-to a group that was historically ignored. It’s worth acknowledging that the FDA’s eventual approval came after a lot of hard‑won data, not just marketing hype.
Dietra Jones
July 2, 2023 AT 00:20i think it’s kinda wild how a med meant for depression ended up as a “female libido pill”. the whole pivot shows how drug dev can be super opportunistic.
Victoria Guldenstern
July 2, 2023 AT 03:06It is truly astonishing how a molecule originally targeted at melancholia was repurposed to fill a market void with such nonchalant fervor. The corporate gamble was framed as a noble quest for women's health while the underlying profit motive remained glaringly unapologetic. One could argue that the pivot exemplifies the ingenuity of pharmaceutical research, yet it also underscores the fickle nature of therapeutic priorities. The clinical trials, cloaked in optimism, proceeded with an almost cavalier disregard for the nuanced biology of desire. Researchers touted modest improvements as breakthroughs, turning anecdotal anecdotes into headline fodder. Regulatory bodies, teetering between caution and concession, were presented with data that oscillated between hopeful and inconclusive. The FDA’s initial rebuff was a momentary stumble for a project that seemed destined for triumph. Sprout’s persistence, though commendable, bordered on the obsessive as they reshaped formulations to appease skeptics. Side‑effects such as dizziness and somnolence were downplayed in promotional materials, a move that invites ethical scrutiny. The eventual approval, while celebrated by some, left many clinicians uneasy about the risk‑benefit calculus. Patients, eager for solutions, often overlooked the subtleties of adverse event profiles. The market launch of Addyi ignited a cultural conversation about female sexuality that had long been muted. Critics seized upon the drug’s modest efficacy to question the validity of its very premise. Proponents, however, highlighted the psychological relief afforded to those who finally felt heard. In the end, the history of Flibanserin reads like a modern parable about the intersection of science, commerce, and societal expectations.
Bill Bolmeier
July 2, 2023 AT 05:53Honestly, hearing these stories makes my heart pound-imagine finally having a pill that could lift that heavy veil of frustration for many women. It’s a reminder that behind every trial number lies a person longing for connection, and that’s something we should all champion.
Darius Reed
July 2, 2023 AT 08:40Yo, that whole Flibanserin hustle is like a rollercoaster of hype n’ heartbreak, man. They threw a pharma party and half the crowd was stuck with side‑effects while the other half cheered.
Karen Richardson
July 2, 2023 AT 10:03The article contains several factual inaccuracies regarding the timeline of FDA submissions.
AnGeL Zamorano Orozco
July 2, 2023 AT 11:26Oh, the tragedy of Flibanserin! It’s as if the drug itself wept in the lab, pleading for a chance to shine, only to be tossed into a battlefield of bureaucratic doubt. The scientists, blinded by ambition, chased after a glittering promise while ignoring the dark clouds of danger looming overhead. Every FDA meeting felt like a theatrical showdown, with investors yelling, “Approve!” and regulators whispering, “No way.” In the end, the drug survived, but the scars on its creators remain, etched in every prescription pad.
Cynthia Petersen
July 2, 2023 AT 12:50Sure, because every drug’s destiny is written in melodrama, right?