When you pick up a prescription, you might see two options: the brand-name pill you’ve heard of, or a cheaper generic version with a different color and shape. Many people wonder-does the generic really work the same? The answer isn’t guesswork. It’s science, enforced by the U.S. Food and Drug Administration (FDA) through one of the most rigorous drug approval systems in the world.
What Makes a Generic Drug ‘The Same’?
A generic drug isn’t just a copy. It has to be therapeutically equivalent to the brand-name version. That means it must contain the same active ingredient, in the same strength, and deliver it to your body the same way. The FDA doesn’t accept ‘close enough.’ It demands proof.
Every generic drug must match the brand-name drug in three critical ways:
- Active ingredient: Exactly the same chemical compound. No exceptions.
- Dosage form: Tablet, capsule, liquid, injection-same format, same route (oral, topical, etc.).
- Strength: Within ±5% of the brand-name drug’s labeled amount.
Inactive ingredients (like fillers, dyes, or preservatives) can differ. But even those are checked. The FDA’s Inactive Ingredient Database sets safe limits for over 500 excipients across 80 different delivery methods. If a generic uses a new excipient, the manufacturer must prove it’s safe at that dose.
The Bioequivalence Test: How the FDA Proves It Works the Same
The real test isn’t about chemistry-it’s about what happens inside your body. That’s where bioequivalence comes in.
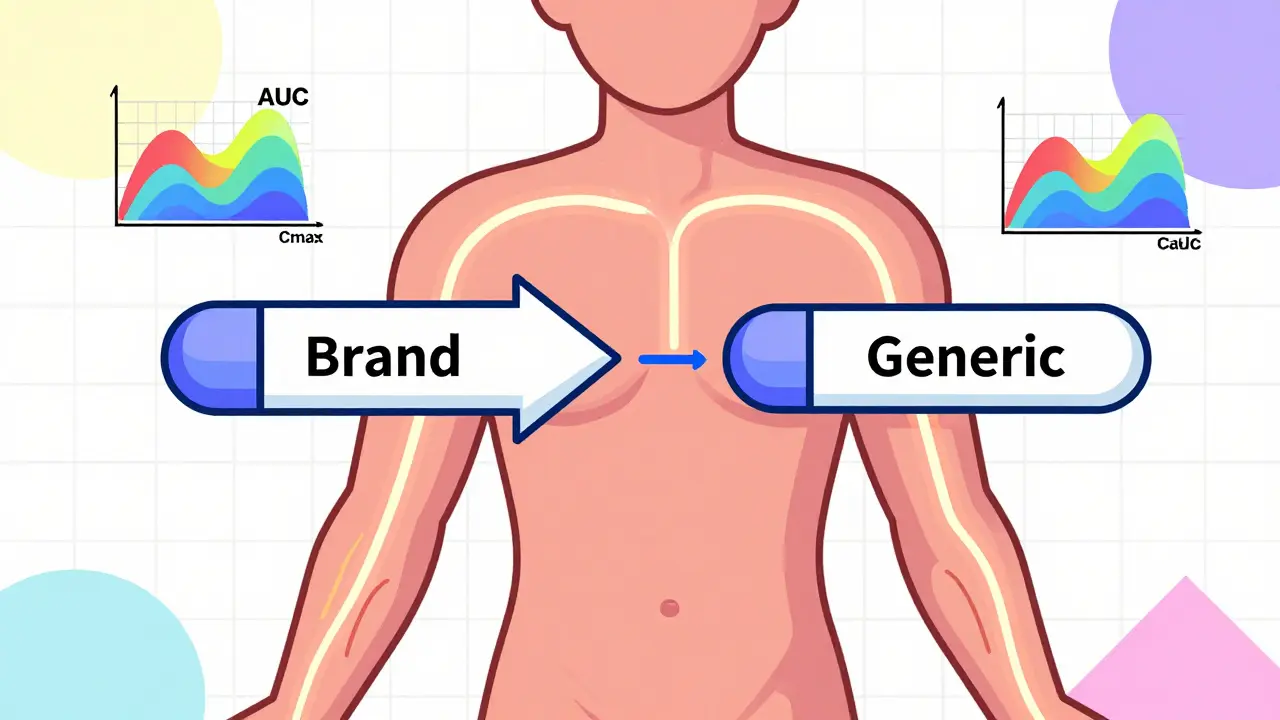
To get approved, a generic drug must show that it absorbs into your bloodstream at the same rate and to the same extent as the brand-name version. This is measured using two key numbers:
- AUC (Area Under the Curve): Total amount of drug absorbed over time.
- Cmax (Maximum Concentration): Peak level of drug in your blood.
The FDA requires that the 90% confidence interval for the ratio of generic to brand-name values for both AUC and Cmax falls between 80% and 125%. That’s not a wide range-it’s a tight scientific window. If a generic drug’s absorption profile falls outside this range, it’s rejected.
These tests are done in clinical studies with 24 to 36 healthy volunteers. They fast overnight, take the drug, and have their blood drawn repeatedly over 24-72 hours. The data is analyzed statistically. No shortcuts. No assumptions.
For complex drugs like inhalers, topical creams, or injectables, the requirements are even stricter. The FDA has special guidelines for these, and some require additional testing beyond standard bioequivalence.
The ANDA Process: The Shortcut That Isn’t a Shortcut
Brand-name drug makers spend years and billions on clinical trials to prove safety and effectiveness. Generic makers don’t repeat that. Instead, they file an Abbreviated New Drug Application (ANDA). The word ‘abbreviated’ doesn’t mean ‘easier.’ It means they rely on the FDA’s existing knowledge of the brand-name drug.
The ANDA still requires:
- Full chemistry and manufacturing data (CMC)
- Proof of bioequivalence
- Identical labeling
- Compliance with Current Good Manufacturing Practices (cGMP)
Manufacturing facilities are inspected-just like brand-name plants. In 2022, 21% of rejections (called Complete Response Letters) were due to manufacturing issues. That’s not a paperwork glitch. It’s a quality failure.
The FDA targets a 10-month review timeline for standard ANDAs. In 2022, they hit 91.3% of that goal. But it’s not just speed-it’s precision. Of the ANDAs submitted that year, 35% were refused outright for being incomplete or flawed.
Why Some Doctors Still Have Concerns
For most drugs, the FDA’s system works flawlessly. But there’s one group where even small differences matter: narrow therapeutic index (NTI) drugs. These are medications where a tiny change in blood level can cause harm or treatment failure.
Examples include:
- Warfarin (blood thinner)
- Levothyroxine (thyroid hormone)
- Phenytoin (seizure control)
- Cyclosporine (organ transplant)
In 2019, the FDA created a special list for these drugs and tightened bioequivalence standards to 90-111% instead of 80-125%. Still, a 2020 study in JAMA Internal Medicine found that 11% of physicians reported clinical concerns when switching patients on NTI drugs to generics-even when the FDA had approved them.
The FDA doesn’t ignore this. They’ve responded with more detailed guidance, stricter testing, and post-market monitoring. But the point remains: for these drugs, doctors sometimes prefer to stick with one version.
Real-World Results: Do Generics Actually Work?
Lab data is one thing. Real patients are another. What happens when millions switch?
A 2023 analysis of 15 million patient records by IQVIA found no meaningful difference in outcomes between brand and generic versions of 20 common drugs-including atorvastatin (for cholesterol) and metformin (for diabetes). In fact, adherence was 3.2% higher with generics. Why? Because they cost less.
The FDA’s own adverse event database (FAERS) shows identical rates of side effects per million prescriptions: 1.6 for brand-name drugs, 1.7 for generics. No difference.
On Reddit’s r/pharmacy, 82% of pharmacists said they’ve never seen a real clinical difference between approved generics and brands. But 18% noted rare complaints-usually with complex products like inhalers or topical creams. Patients sometimes say, ‘This one doesn’t work as well.’ But when tested, the drug levels are the same.
That’s where perception comes in. A 2021 survey found 37% of pharmacists had patients who doubted generics. But the FDA’s patient surveys show 89% trust them. The disconnect? Often, it’s about packaging, size, or color-not chemistry.
Who Makes These Drugs-and Why It Matters
Most people think generics are made in cheap overseas labs. That’s partly true. But the FDA inspects all facilities-whether they’re in the U.S., India, or China. In 2022, nearly half of all generic drug manufacturing sites were outside the U.S. And they’re held to the same cGMP standards.
Big players like Teva, Viatris, and Sandoz dominate the market. But over half of all generic approvals go to smaller companies. Why? The FDA’s Generic Drug Competition Action Plan pushes for more suppliers to prevent shortages. When only one company makes a drug, prices can spike. When five do, they compete.
Developing a generic drug costs $1.5 million to $3 million on average. For complex products like generic EpiPens, it can hit $25 million. The paperwork? 30,000 to 50,000 pages. The bioequivalence section alone? Up to 10,000 pages of raw data.
And then there’s the patent maze. Generic makers often challenge patents under Paragraph IV of the Hatch-Waxman Act. If they do, the brand-name company can sue, triggering a 30-month legal hold. That’s why some generics take years to hit the market-even after approval.
What’s Next for Generic Drugs?
The FDA isn’t resting. With over $260 billion in brand-name drug revenue set to lose patent protection between 2024 and 2028, they’re preparing for a flood of applications. Their new GDUFA III plan aims to cut review times to 8 months for standard ANDAs and 6 months for priority ones.
They’re also launching a new pathway for biosimilars-generic versions of complex biologic drugs like Humira and Enbrel. The first 15 product-specific guidances are coming in 2024.
And they’re speeding up approvals for drugs in short supply. In 2023, 47 drugs got fast-track status. Twelve were approved in under six months-half the normal time.
The message is clear: the FDA’s system isn’t perfect, but it’s robust, transparent, and constantly improving. Generics aren’t second-rate. They’re rigorously tested, scientifically validated, and clinically proven to be the same.
When you choose a generic, you’re not choosing a bargain. You’re choosing a drug that passed the same test as the brand-and saved you hundreds of dollars in the process.
Are generic drugs as safe as brand-name drugs?
Yes. The FDA requires that generic drugs meet the same safety and quality standards as brand-name drugs. All manufacturing facilities, whether in the U.S. or overseas, must pass the same inspections and comply with Current Good Manufacturing Practices (cGMP). Adverse event reports show identical rates between generics and brands-1.7 per million prescriptions for generics versus 1.6 for brand-name drugs.
Why do generic pills look different from brand-name ones?
By law, generics can’t look identical to brand-name drugs, even if they contain the same active ingredient. This is to avoid confusion and trademark infringement. Differences in color, shape, or size are due to inactive ingredients or design choices-not effectiveness. The drug’s performance in your body is unchanged.
Can I switch between different generic brands?
Yes, and it’s common. Every generic approved by the FDA must meet the same bioequivalence standards. So switching between different generic manufacturers-say, from one company’s metformin to another’s-is safe. The active ingredient, strength, and absorption rate are identical. If you notice a change in how you feel, talk to your doctor-but it’s rarely due to the drug itself.
Are all generic drugs FDA-approved?
Only those sold in the U.S. market and labeled as FDA-approved are. Some online sellers offer ‘generic’ drugs from unregulated sources-these are illegal and unsafe. Always check the label for the manufacturer’s name and confirm the drug is listed in the FDA’s Drugs@FDA database. If it’s not approved by the FDA, it hasn’t been tested for safety or effectiveness.
Do generics take longer to work than brand-name drugs?
No. The FDA requires that generics reach the same peak concentration in the bloodstream (Cmax) within the same timeframe as the brand-name drug. Bioequivalence studies measure absorption speed and total exposure (AUC). If a generic were slower to work, it would fail approval. Any perceived delay is likely due to individual metabolism, food intake, or placebo effect-not the drug.
Why are generics so much cheaper?
Generics are cheaper because they don’t repeat the expensive clinical trials that brand-name drugs undergo. Instead, they rely on the FDA’s prior approval of the original drug. Development costs for a generic are typically $1.5 million to $3 million, compared to over $1 billion for a new brand-name drug. With multiple manufacturers competing, prices drop further-saving the U.S. healthcare system over $300 billion a year.
What to Do If You’re Unsure About a Generic
If you’ve been switched to a generic and feel different, don’t assume the drug is faulty. Talk to your pharmacist first. They can check if the generic you’re getting is on the FDA’s approved list and whether it’s the same version you used before.
If you’re on a narrow therapeutic index drug-like warfarin or levothyroxine-ask your doctor if sticking with one brand or generic is right for you. Consistency matters more than cost in these cases.
And if you’re worried about quality, remember: every generic sold legally in the U.S. has passed the same inspection and testing as the brand. The FDA doesn’t cut corners. They just cut the cost.

15 Comments
Aysha Siera
January 18, 2026 AT 11:41The FDA is just another puppet of Big Pharma. They approve generics so you think you're saving money but the real profit is in the inactive ingredients that make you sick later. They don't want you to know this.
They change the dye. The dye is the key. The dye is what makes you dependent. You think you're getting the same drug? You're getting a slow poison with a different label.
Andrew McLarren
January 18, 2026 AT 19:57While the FDA's regulatory framework for generic drugs is indeed robust and grounded in rigorous scientific methodology, it is imperative to acknowledge that the translation of bioequivalence metrics into clinical equivalence remains a nuanced domain. The statistical thresholds of 80-125% for AUC and Cmax, while mathematically sound, may not fully account for inter-individual pharmacokinetic variability, particularly in populations with comorbidities or polypharmacy.
Andrew Short
January 19, 2026 AT 19:17You people are delusional if you think this system works. The FDA is a joke. They approve generics from factories in India that have been cited for data falsification. They don't inspect properly. They just rubber stamp. And you swallow it like it's medicine. Pathetic.
christian Espinola
January 19, 2026 AT 20:13The 80-125% bioequivalence range is statistically absurd. A 25% variance in absorption? That's not equivalence, that's a lottery. And you call this science? The FDA's own data shows 35% of ANDAs get rejected for being incomplete. So if they can't even get the paperwork right, why should we trust the bioequivalence data? The math doesn't add up. The system is broken.
Naomi Keyes
January 21, 2026 AT 10:32I just want to say, as someone who's been on levothyroxine for 12 years, I switched generics four times last year alone-and each time, my TSH went haywire. I had to go back to the brand. And yes, it cost me $200 a month. But my body doesn't care about cost. My body cares about stability. The FDA says it's 'equivalent,' but my symptoms say otherwise. And I'm not crazy. I'm just tired of being told I'm imagining it.
Dayanara Villafuerte
January 21, 2026 AT 16:43Y'all need to chill 😌 The FDA isn't perfect, but it's the best we've got. I work in pharmacy and I've seen 1000s of generic switches. The only time people notice a difference? When they're stressed, or they just don't like the new pill shape. 💊✨ The science is solid. Trust the data, not your anxiety.
Jodi Harding
January 23, 2026 AT 03:19They test it in 30 healthy people who don't have chronic illness. Then they say it's safe for everyone. That's not science. That's assumption.
Danny Gray
January 24, 2026 AT 21:11What if the real problem isn't the drug... but the fact that we've been conditioned to believe that cheaper = worse? Maybe the discomfort we feel isn't from the medication-it's from the story we tell ourselves about it. The pill doesn't change. Our perception does.
Zoe Brooks
January 25, 2026 AT 03:05I switched to generic metformin and my blood sugar dropped faster. I didn't even know that was possible. I thought generics were just cheaper copies. Turns out, they're just... different. Not worse. Just different. And that's okay. 💪
Kristin Dailey
January 25, 2026 AT 17:43If you're not American, you don't get to complain. We have the best drug system in the world. Stop whining about generics. You want quality? Pay for it.
Wendy Claughton
January 26, 2026 AT 12:29I just want to say... I love how much care the FDA puts into this. 🥺 I know it's not perfect, but they're trying. And honestly? I'm so grateful that I can afford my meds now. I used to skip doses because of cost. Now I take them. Every day. And I feel better. Thank you, FDA. You're doing important work.
Stacey Marsengill
January 27, 2026 AT 06:30The FDA is a cult. They worship data and ignore human experience. You think your 90% confidence interval means something? Try living with a thyroid condition where your hands shake because the generic made you hypo. Then come back and tell me about 'equivalence'. The system doesn't see you. It sees a number.
Jay Clarke
January 27, 2026 AT 11:23I'm not saying the FDA is lying. I'm saying they're clueless. You can't measure a human being with a blood test. You think Cmax means anything when someone's stressed, or sleeping poorly, or eating junk? No. You're measuring a ghost. The drug works the same? Maybe. But the person? Not anymore.
Selina Warren
January 29, 2026 AT 09:27Stop acting like generics are dangerous. They're not. They're the reason millions of people can afford to live. If you're scared of them, you're scared of progress. Wake up. This isn't a conspiracy-it's capitalism working for the people. Stop being afraid of saving money.
Eric Gebeke
January 31, 2026 AT 04:25I've been on warfarin for 15 years. I've used six different generics. My INR has never been off by more than 0.3. The FDA is right. The science is real. The fear is manufactured. I used to be paranoid too. Then I read the studies. Now I take the generic. And I'm still alive.