The FDA generic approval timeline isn’t a single number-it’s a range shaped by drug complexity, application quality, and agency resources. If you’re waiting for a generic version of a brand-name drug to hit the market, you’re likely wondering: How long am I really looking at? The answer isn’t simple, but it’s not mysterious either. Here’s what actually happens from submission to approval, based on the latest data from 2025 and early 2026.
What’s the Standard Timeline?
The FDA’s official goal for a standard generic drug application (called an ANDA) is 10 months after the application is accepted. That’s the clock that starts ticking once the agency confirms your paperwork is complete and ready for review. But that’s the target, not the average.
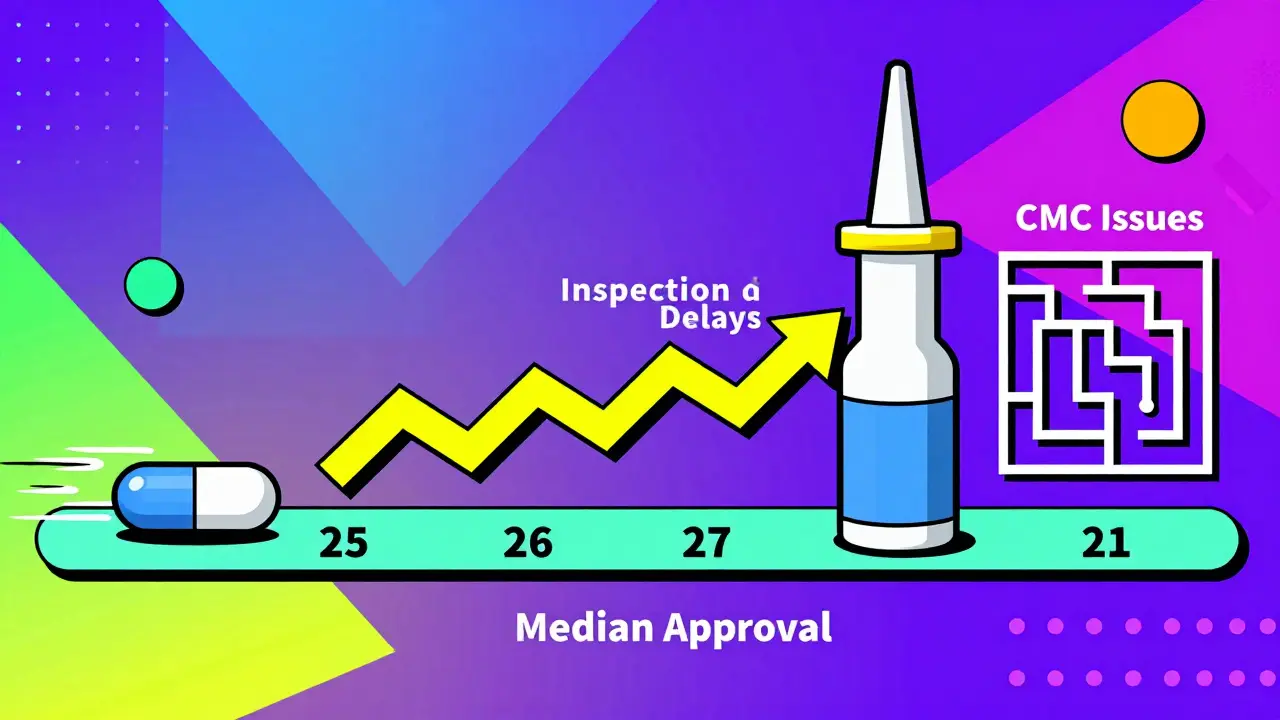
In reality, the median approval time-meaning half of all approvals happen faster, half take longer-dropped to 25.3 days in Q4 2025. That’s down from nearly 26 days the quarter before, and from 26.2 days in early 2024. The mean (average) time is longer: around 35.6 days. Why the gap? Because a small number of complex applications drag the average up.
So if you’re making a simple tablet-like generic ibuprofen or metformin-you’re likely looking at 1 to 3 months from acceptance to approval. But if your drug is a nasal spray, injectable, or one with a tricky delivery system? That’s a different story.
Why Do Some Approvals Take Months Longer?
Not all generics are created equal. The FDA categorizes them as “simple” or “complex.” Simple means a standard oral tablet or capsule with well-understood chemistry. Complex means anything that’s harder to copy: extended-release pills, inhalers, patches, injectables, or drugs with multiple active ingredients.
For complex generics, review times can stretch to 6 to 12 months. One Reddit user shared their experience: a generic nasal spray took 1,087 days from submission to approval. That’s almost three years. Why? Because the FDA has to verify the drug behaves the same way in the body as the brand-name version-not just in a test tube, but in real human physiology. That’s harder to prove with complex delivery systems.
Another big delay comes from incomplete applications. If your chemistry, manufacturing, or controls (CMC) section is missing data, or if your bioequivalence study doesn’t meet FDA standards, you’ll get a complete response letter. This isn’t a rejection-it’s a list of fixes. Addressing it usually adds 3 to 6 months. In Q2 2025, 42.3% of all complete response letters came in the first review cycle, meaning the FDA is catching issues earlier. That’s good news-if you fix it right the first time.
What About Priority Review?
If a generic drug treats a condition with no other available version-or if there’s a shortage-the FDA can give it priority review. There’s no official deadline for priority generics, but the agency aims to move them faster. In practice, priority applications often clear review in 6 to 8 months, sometimes even less.
The FDA has also started using AI to speed things up. In pilot programs during 2024, AI tools helped reduce review times for standard generics by 15.8%. The agency is now testing AI to flag potential issues in application documents before human reviewers even open them. That’s not science fiction-it’s happening right now.
Even more dramatic: the new Commissioner’s National Priority Voucher (CNPV) program, launched in late 2025, can cut approval time from 10 months to just 1 to 2 months for select applications. These are reserved for generics that address critical shortages or treat rare diseases. Only a handful get this treatment each year-but it shows how fast the system can move when it needs to.

How the Process Actually Works
Here’s the step-by-step breakdown of what happens after you submit your ANDA:
- Filing Review (up to 60 days): The FDA checks if your application is complete. Missing forms? Incomplete data? They’ll send you a deficiency letter. You have to fix it before the clock starts.
- Review Clock Starts: Once accepted, the 10-month goal begins. Reviewers examine chemistry, manufacturing, bioequivalence, labeling, and facility inspections.
- Pre-Approval Inspection: The FDA inspects your manufacturing site. If you’re new to the U.S. market, this can cause delays-especially if your facility is overseas.
- Complete Response Letter (if needed): You get a list of issues. You respond, and the clock resets. Most companies need 1 to 2 cycles.
- Approval: The drug is cleared for sale. You’ll get an approval letter and a new entry in the FDA’s Orange Book.
Many manufacturers don’t realize that how you submit matters as much as what you submit. Companies that use the FDA’s pre-submission meeting program-where they talk to reviewers before filing-see approval times that are 20% faster on average. Teva, Viatris, and Sandoz, the top three generic makers, use this process religiously.
Who’s Getting Approved Fastest?
Market leaders aren’t just bigger-they’re smarter about the process. Teva Pharmaceutical leads with 18.3% of all approved generics in 2025, followed by Viatris (14.2%) and Sandoz (11.7%). Their secret? Consistent communication with the FDA, high-quality submissions, and deep experience with complex products.
Small companies and first-time applicants often struggle. Only 4.7% of ANDA submissions qualify for fee waivers, but even those with waivers can get approval quickly if their application is flawless. The FDA’s Complex Generic Drug Products initiative, launched in 2023, assigns dedicated teams to tough-to-copy drugs. Since then, approval times for these products have dropped by 22%.

Why This Matters for Patients
Every generic drug approved saves money. In 2025, generic drugs made up 90% of all prescriptions in the U.S.-but only 23% of total drug spending. Over the last decade, they’ve saved the healthcare system $1.7 trillion.
When the FDA approves a first generic for a brand-name drug like Humira or Enbrel, the price often drops by 80% or more within months. That’s why the agency prioritizes “first generics.” In late 2025, first generic approvals were pacing ahead of 2024 levels, while new brand-name drug approvals slowed slightly. That’s intentional: the public gets more value from generics than from new brand drugs, especially when the brand drug is already expensive.
But there’s a catch. Some experts warn that rushing approvals could risk quality. Dr. Peter Lurie of the Center for Science in the Public Interest said in August 2025: “Rushing without adequate oversight could mean patients get a generic that doesn’t work the same.” The FDA responds by saying it uses a risk-based approach, focusing more scrutiny on drugs with higher safety risks or no competition.
What’s Coming Next?
The FDA’s next phase, under GDUFA III (2023-2027), aims for even faster approvals. By 2027, the goal is to hit a median approval time of just 20 days for standard generics and 10 days for priority ones. That’s ambitious. To get there, the agency will need more staff, better tech, and stable funding.
Right now, the system is working-but unevenly. If you’re a manufacturer, your best move is to submit a clean, complete application. If you’re a patient waiting for a cheaper version of your medication, you’re likely looking at 2 to 6 months for a simple drug, and up to a year or more for complex ones. The good news? The trend is clear: generics are getting approved faster than ever.
What You Can Do
If you’re a patient or caregiver:
- Check the FDA’s Approved Drug Products with Therapeutic Equivalence Evaluations (Orange Book) for new generic approvals.
- Sign up for FDA email alerts on generic drug approvals.
- If your drug is in shortage, ask your pharmacist about alternatives-they may know of a recently approved generic.
If you’re a manufacturer:
- Use pre-submission meetings-even if you’re small.
- Invest in high-quality CMC data. Don’t cut corners.
- Don’t wait until the last minute to prepare for inspections.
- Consider applying for priority review if your drug addresses a shortage or has no competition.
How long does it take the FDA to approve a generic drug?
The FDA’s goal is 10 months from acceptance of the application. But the median approval time in late 2025 was just 25.3 days. Simple generics often clear approval in 2 to 6 months. Complex ones-like injectables or extended-release formulations-can take 6 to 12 months or longer, especially if the application needs revisions.
What’s the difference between a standard and priority FDA review for generics?
Standard review targets a 10-month timeline and applies to most generics. Priority review is for drugs that treat serious conditions with no other options or are in short supply. While there’s no fixed deadline, priority applications typically move through the system in 6 to 8 months. The new Commissioner’s National Priority Voucher program can cut this to just 1-2 months for the most urgent cases.
Why do some generic approvals take over a year?
Complex formulations-like nasal sprays, patches, or multi-component drugs-require more testing to prove they work the same as the brand. Delays also happen if the application is incomplete, the manufacturing site needs inspection, or the FDA issues a complete response letter requiring fixes. One user reported a 1,087-day approval for a nasal spray generic due to these factors.
Can the FDA approve a generic drug faster than 10 months?
Yes. In 2025, the median approval time was under 26 days. The FDA’s AI tools, pre-submission meetings, and the new Priority Voucher program are helping cut review times dramatically. Some simple generics are approved in as little as 60 days. The goal is to reach a median of 20 days by 2027.
How do I know if a generic drug has been approved?
Check the FDA’s Orange Book, which lists all approved generic drugs and their therapeutic equivalence ratings. You can search by brand name or active ingredient. The FDA also sends out email alerts for new approvals. Pharmacies and insurance providers update their formularies within days of approval.
Are there fees to apply for FDA generic approval?
Yes. The standard user fee for an ANDA in fiscal year 2025 was $138,400. Small businesses and first-time applicants may qualify for fee waivers, but only about 4.7% of applications use them. The fee helps fund the FDA’s review process and has been key to improving approval speeds under the Generic Drug User Fee Amendments (GDUFA).

1 Comments
Shweta Deshpande
January 26, 2026 AT 05:32Wow, I had no idea the median approval time was under 26 days now! I always thought it took forever. My dad’s blood pressure med just got generic last month and it cut our copay in half-felt like winning the lottery. The FDA’s actually doing something right for once 🙌