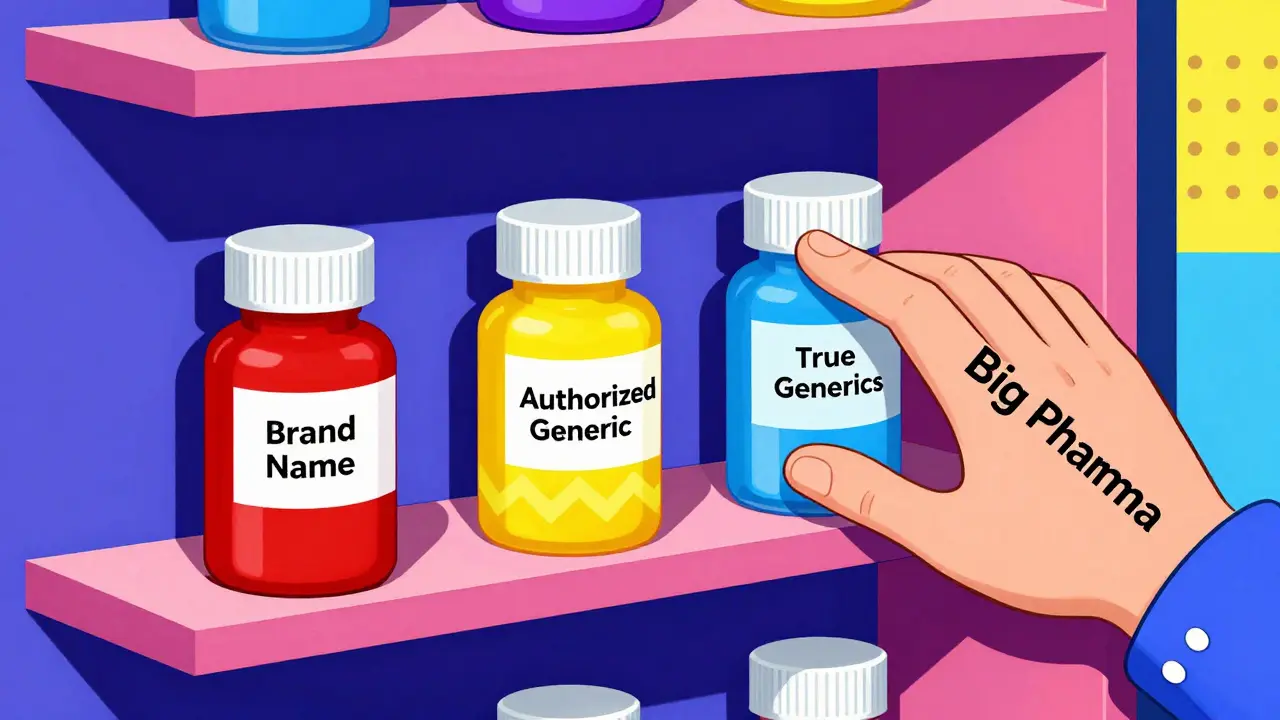
Authorized Generics: What They Are and Why They Matter for Your Health
When you hear authorized generics, brand-name drugs sold under a generic label with the exact same ingredients, manufacturing, and quality control as the original. Also known as brand-name generics, they’re not knockoffs—they’re the same pills, made in the same factory, often by the same company that made the brand version. This isn’t marketing fluff. It’s how the system is supposed to work: when a drug’s patent expires, the original maker can legally produce and sell its own generic version, keeping quality high while lowering the price.
Why does this matter? Because not all generics are created equal. Some are made by third-party labs, and while they’re FDA-approved, they might use slightly different fillers or coatings. authorized generics, are identical to the brand drug in every way, down to the inactive ingredients. This means no surprise reactions, no inconsistent absorption, and no guesswork when switching from brand to generic. For people on long-term meds—like blood pressure pills, antidepressants, or thyroid hormones—that consistency can mean the difference between feeling stable and feeling off.
And here’s the kicker: authorized generics are often cheaper than regular generics because they’re sold directly by the brand manufacturer, cutting out middlemen. You might pay half the price of the brand name, even less than other generics, and still get the exact same product. That’s not a loophole—it’s a feature of the system designed to increase competition.
Some people still think generics are "inferior" because they look different or cost less. But if you’ve ever been prescribed an authorized generic, you’re getting the same tablet your doctor originally wrote for, just without the fancy packaging and marketing costs. It’s like buying the same coffee beans in a plain bag instead of a branded box. Same roast, same flavor, same caffeine—just less money spent on ads.
The real question isn’t whether authorized generics work—it’s why more people don’t ask for them. Pharmacists know about them. Insurance plans often favor them. But unless you ask, you might not even know you’re getting a brand-name version when a cheaper, identical option is right there.
That’s why the posts below cover everything from how to spot an authorized generic on your prescription label, to why some drug companies hide them behind complex patent tricks, to how pharmacists help you save hundreds a year by switching you to the real thing. You’ll also find real stories from people who switched and never looked back—and others who got burned by fake generics that weren’t authorized at all.
Patent Litigation: How Authorized Generics Undermine Generic Drug Competition
Authorized generics let brand drugmakers launch their own versions alongside true generics, undermining the 180-day exclusivity meant to reward patent challengers. This practice reduces competition, delays price drops, and distorts the market.
180-Day Exclusivity and Authorized Generics: Legal Considerations in U.S. Drug Markets
The 180-day exclusivity rule was meant to reward generic drug makers for challenging patents. But brand-name companies can legally launch their own versions - called authorized generics - during that window, undercutting profits and undermining the system’s purpose.
© 2026. All rights reserved.