Hatch-Waxman Act: How It Shaped Generic Drugs and Drug Prices
When you pick up a generic pill at the pharmacy and save half the price of the brand-name version, you’re seeing the direct result of the Hatch-Waxman Act, a 1984 U.S. law that balanced drug innovation with affordable access. Also known as the Drug Price Competition and Patent Term Restoration Act, it created the modern system for generic medications in America. Before this law, drug companies could block generics indefinitely by holding patents and delaying approval—even if the original drug’s formula had long expired. Hatch-Waxman changed that by letting generic makers prove their versions were just as safe and effective, without repeating expensive clinical trials.
But it wasn’t just about generics. The Act also gave brand-name drugmakers a way to extend their patents, by adding up to five years of market exclusivity to make up for time lost during FDA review. That’s why you see some drugs stay expensive for 12, 15, even 20 years—companies file new patents on minor changes like coating, dosage, or delivery method. These are called secondary patents, legal tools used to delay generic competition, and they’re at the heart of today’s drug pricing debates. You’ll see this play out in posts about how brands stretch exclusivity, how biosimilars differ from generics, and why some medications still cost hundreds even after the original patent expires.
The Hatch-Waxman Act didn’t just affect drug prices—it shaped how pharmacies operate, how doctors prescribe, and how patients access care. It’s why pharmacists can confidently recommend generics to save money. It’s why counterfeit drugs are harder to pass off as real—because the Act set clear standards for approval. And it’s why biologics, complex drugs made from living cells, still face delays in generic-style competition today. The system it built is still running, but it’s under pressure. Posts here break down how patent thickets work, how biosimilars are approved differently, and why some patients still pay more than they should.
What follows is a collection of real-world stories about how this law still impacts your prescriptions today—from the pills you take for depression, arthritis, or heart disease, to the hidden costs buried in patent extensions and the quiet battles between pharmacies and drugmakers over what’s truly interchangeable. You’ll find out why some generics take years to appear, how companies fight to keep prices high, and what you can do to make sure you’re getting the best deal.
30-Month Stay: How Litigation Delays Generic Drug Approval
The 30-month stay under the Hatch-Waxman Act is meant to balance patent rights and generic access - but it often delays affordable drugs for years. Learn how litigation, patent tricks, and corporate deals keep prices high.
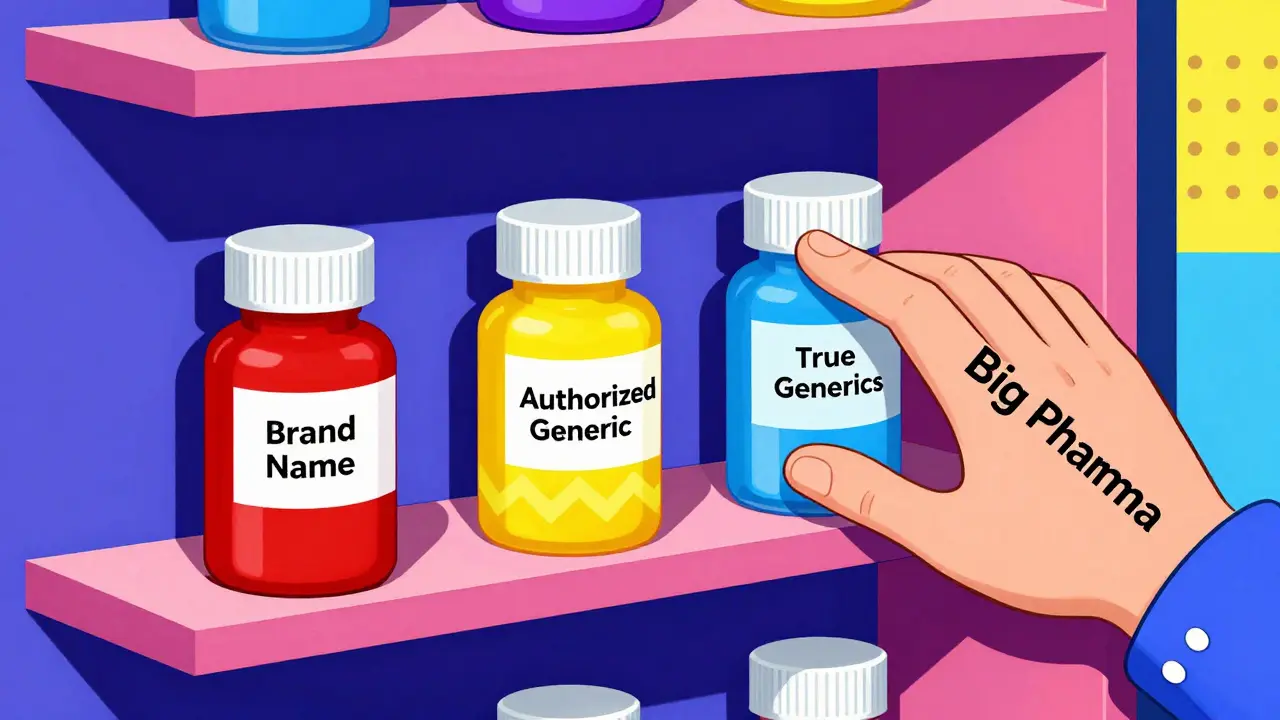
Patent Litigation: How Authorized Generics Undermine Generic Drug Competition
Authorized generics let brand drugmakers launch their own versions alongside true generics, undermining the 180-day exclusivity meant to reward patent challengers. This practice reduces competition, delays price drops, and distorts the market.
180-Day Exclusivity and Authorized Generics: Legal Considerations in U.S. Drug Markets
The 180-day exclusivity rule was meant to reward generic drug makers for challenging patents. But brand-name companies can legally launch their own versions - called authorized generics - during that window, undercutting profits and undermining the system’s purpose.
© 2026. All rights reserved.